Crown-Ether Chiral Stationary Phases
CrownSil™ crown ether columns are ideal for the separation of chiral compounds containing primary amines and natural and unnatural amino acids and their derivatives. Other racemic compounds, such as amino alcohols (β -blockers), secondary amines, drugs containing primary amines and secondary amines can also be resolved on CrownSil™ columns.
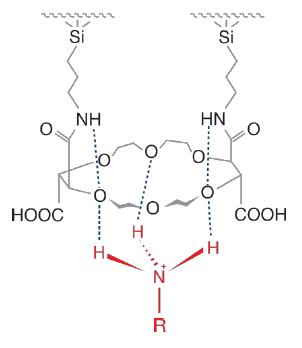
The chiral stationary phase for CrownSil™ R(+) and S(-) is prepared by covalently bonding of (+) or (-)-(18-Crown-6)-tetracarboxylic acid to an aminopropyl silica gel support, ensuring excellent column durability. The CrownSil™ CSP is compatible with both normal phase and reversed-phase high-performance liquid chromatography (HPLC) and is available in both analytical and preparative dimensions. It is also available in both enantiomeric forms (R(+) or S(-)), which allows for inversion of peak elution order, which can be beneficial for preparative separations. For amino acids, most L-enantiomers elute first on the CrownSil™ -R(+) and D-enantiomers elute first on the CrownSil™ -S(-) column.
The mechanism of separation is two-fold:- Complexation of the primary ammonium group (R-NH 3+) formed by protonation of α-amino acids and primary amines under acidic conditions inside the cavity of the 18-crown-6 ring of the CrownSil™ CSP
- Side carboxylic acid groups of CrownSil ™ CSP can act as steric barrier groups or as hydrogen bonding donor or accepter groups
CrownSil™ Selector Structure
Looking for ChiroSil® columns? Connect with a member of our sales team to place an order.
Resources
View our Chiral Applications Database.
Request or download a free copy of our Chiral Handbook that contains more than 950 chiral separations.
Email Us for more information.
CrownSil™ is a trademark of CellaChrom™. CellaChrom™ is a trademark of Cellion BioMed Inc.

