Why You Should Leverage Pharmaceutical Solid State Chemistry Early in Drug Development


 Pharmaceutical solid state chemistry – the study of functional relationships between the synthesis, structure, and properties of solid phase materials – plays a crucial role in successful drug development and commercialization. As active pharmaceutical ingredients (APIs) have grown more complex, the importance of understanding the polymorphic landscapes have, as well. Polymorphs are created when an API organizes itself in different crystalline arrangements – whether as an anhydrous species, a hydrated species, or a solvated species.
Pharmaceutical solid state chemistry – the study of functional relationships between the synthesis, structure, and properties of solid phase materials – plays a crucial role in successful drug development and commercialization. As active pharmaceutical ingredients (APIs) have grown more complex, the importance of understanding the polymorphic landscapes have, as well. Polymorphs are created when an API organizes itself in different crystalline arrangements – whether as an anhydrous species, a hydrated species, or a solvated species.
Each of these solid forms introduces unique physical and chemical variations in the compound’s structure and changes the API’s characteristics – potentially impacting its performance and ultimately affecting drug product performance. Since different polymorphs have different physicochemical properties, identifying a viable polymorph early during the drug development process can reduce your costs and shorten the time to market.
The Impact of Polymorphs
The vast majority of organic molecules used as active pharmaceutical ingredients (APIs) exhibit polymorphism. Polymorphs have different physicochemical properties, including:
- spectroscopic
- surface chemistry (solubility and stability)
- crystal habit or morphology (particle shape)
- packing
- kinetic
- thermodynamic
- mechanical
 These properties influence key attributes in drug substances and drug products, such as dissolution rate or bioavailability because polymorphs have different solubilities. Particle size distributions can vary (tighter or wider), which influences drug product manufacturing and performance. They also possess thermodynamic and kinetic differences, which may impact stability.
These properties influence key attributes in drug substances and drug products, such as dissolution rate or bioavailability because polymorphs have different solubilities. Particle size distributions can vary (tighter or wider), which influences drug product manufacturing and performance. They also possess thermodynamic and kinetic differences, which may impact stability.
The application of pharmaceutical solid state chemistry drives understanding of these potential pitfalls, and identifies the optimal polymorph to maximize the success of your drug development efforts.
Early Identification of Viable Polymorphs
Solid state chemistry can identify different polymorphs and characterize the different properties and behaviors. With the growing complexity of drug molecules, applying solid state chemistry practices early on in your development process has become critical in identifying viable polymorphs for further drug development.
The earlier you identify the optimal polymorph, the easier it is to mitigate the risks in drug manufacturing and formulation development.

Building Solid State Knowledge for Regulatory Authorities
By the time you complete Phase II of development, your knowledge about the solid state form of the molecule should be provided to the FDA. This information will include your compound’s propensity to exhibit polymorphism, along with other physicochemical data. At the time you submit an NDA or ANDA, extensive knowledge of the solid state properties of the API must be provided to regulatory agencies. This includes:
- whether the API exhibits polymorphism (i.e., multiple polymorphs exist).
- whether undesired polymorphs affect dissolution and/or bioavailability.
- how various particle size distributions of the material influence dissolution and bioavailability.
- which appropriate test methods for the drug substance and drug product can be used to demonstrate control of the desired polymorph, if necessary.
Because of these requirements, understanding whether your molecule exhibits polymorphism early during development will assist in drug substance manufacturing and in drug product formulation development. In some cases, it may be necessary to develop analytical testing protocols and specifications for relevant polymorphs.
Regulatory Guidance on API Polymorphs
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use – or ICH – is a joint initiative between industry and most regulatory agencies around the world (including the FDA) with the aim of standardizing technical guidelines. The ICH introduced a number of decision trees to guide the industry on polymorphs. (The FDA also published decision trees on polymorphism in its 2007 Guidance for Industry on ANDAs: Pharmaceutical Solid Polymorphism.]
Understanding the physicochemical properties of the polymorphic landscape is essential to appreciate the requirements of ICH Q6A, Decision Tree 4, for example. Below are the ICH’s Q6A’s decision trees, which help drug companies determine what characteristics of the API need to be understood and controlled.
The first decision tree (below) instructs us to conduct a polymorph screen:
Does your molecule exhibit polymorphism?
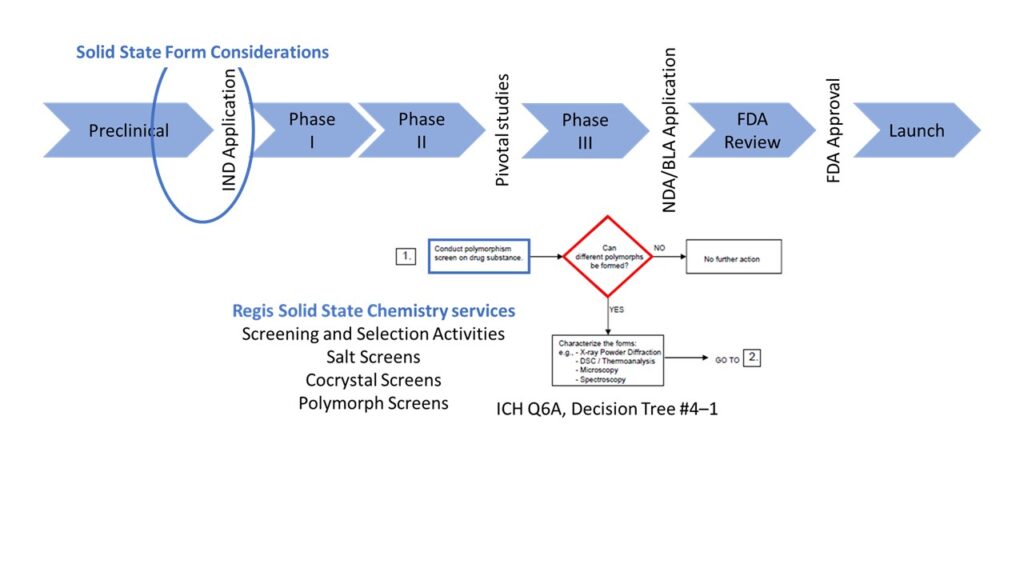
Other decision trees (see example, below) aim to establish whether there is an impact on drug performance or safety:
- Do the different physicochemical properties of the drug substance affect drug product performance, efficacy, or safety? If yes, then establish methods to differentiate between the undesired polymorph(s) and the desired polymorph in the drug substance.
- Does the drug product testing adequately demonstrate control if the ratio between polymorph changes? Monitor the polymorphs in the drug product. If there are changes, does the change affect either safety or efficacy? If yes, establish criteria for the polymorph ratios to guarantee safety and efficacy.
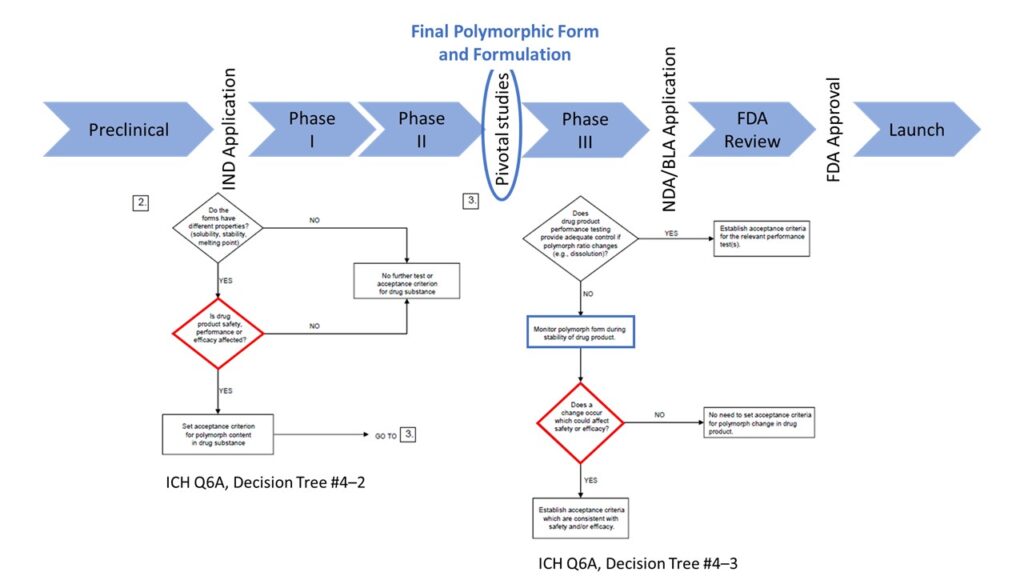
This information will also be vital for support and discussions with the FDA as advised by either the Guidance for Industry, cGMP for Phase 1 Investigational Drugs (solid state stability, pages 10 and 17), or INDs for Phase 2 and Phase 3 Studies, Chemistry, Manufacturing, and Controls Information, pages 13–14.
[For a more in-depth look at how decision trees address polymorphism, check out this short video: Solid State Chemistry Requirements Throughout CMC Development.]
Pharmaceutical Solid State Chemistry & Intellectual Property
Intellectual property rights play a key role in the widespread adoption of solid state chemistry in the pharmaceutical industry. Because polymorphs cannot be reliably predicted, it has become increasingly common during drug development to identify and patent as many polymorphs as possible. The objective of this strategy is to avoid competitive patents later during life cycle management. From a legal perspective, knowledge about polymorphism can be critical, especially to prevent competitors from patenting a polymorph.
There are reports of drug innovators developing a molecule but failing to patent some of the relevant polymorph information. In at least one case, a competitor systematically patented several polymorphs of a novel compound – including the innovator company’s candidate. The competitor then went one step further and sued the innovator company for patent infringement. In another case, a patent for a branded product did not cover all aspects about the ratio between two polymorphs. As a result, a generic version was successfully launched because of this lapse in patent coverage.
Even in the generics industry, such patent strategies have become more commonplace in recent years:
Technologies to Identify and Characterize API Polymorphs
X-Ray Powder Diffraction (XRPD) is a primary technique used today to screen for polymorphs, though other complementary technologies are often employed. At Regis, for example, our solid state chemistry services often involve:
- X-ray Powder Diffraction (variable temperature capabilities)
- Differential Scanning Calorimetry
- Thermal Gravimetric Analysis
- Polarized Light and Hot-stage Microscopy
- Dynamic Vapor Sorption
- Fourier-transform Infrared spectroscopy (FTIR)
Using a wide range of material characterization services, we help our clients build deep knowledge about their solid state forms. Among the most requested services are:
- Screens and Selection Studies for Polymorphs, Salts, and Cocrystals
- Crystallization Process Development
- Technical Assessments and Consultation
- Method Development & Qualification
- Patent Litigation
We urge all our clients to leverage pharmaceutical solid state chemistry early in development. The reasons above – from intellectual property protection to streamlining regulatory submissions and approvals to avoiding scale-up & manufacturing surprises – all support the need for robust understanding of the potential for polymorphs.
Drug API Polymorphs: A Challenging Landscape
Polymorphs continue to present challenges to drug development. Building an understanding of your molecule and its solid forms will mitigate manufacturing and formulation issues, provide intellectual property protection, and demonstrate to regulatory authorities your understanding of your molecule and its solid state forms as it relates to safety and efficacy.
The objective of leveraging pharmaceutical solid state chemistry early in drug development is to develop and implement robust API/formulation processes, mitigate risks, minimize problems, and ultimately reduce development timelines.