|
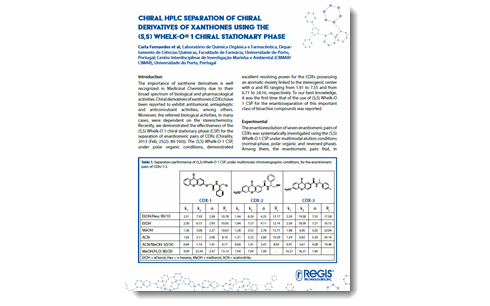
The importance of xanthone derivatives is well recognized in Medicinal Chemistry due to their broad spectrum of biological and pharmacological activities. Chiral derivatives of xanthones (CDXs) have been reported to exhibit antitumoral, antiepileptic and anticonvulsant activities, among others. Moreover, the referred biological activities, in many cases, were dependent on the stereochemistry. Recently, we demonstrated the effectiveness of the (S,S) Whelk-O 1 chiral stationary phase (CSP) for the separation of enantiomeric pairs of CDXs (Chirality, 2013 (Feb; 25(2): 89-100)). The (S,S) Whelk-O 1 CSP, under polar organic conditions, demonstrated excellent resolving power for the CDXs possessing an aromatic moiety linked to the stereogenic center with α and RS ranging from 1.91 to 7.55 and from 6.71 to 24.16, respectively. To our best knowledge, it was the first time that of the use of (S,S) Whelk-O 1 CSP for the enantioseparation of this important class of bioactive compounds was reported. Download |