When Should I Hire a CMC Consultant?


Deciding when to hire a Chemistry, Manufacturing, and Controls (CMC) expert can be difficult. On the one hand, a CMC consultant can supervise your project from start to finish and provide valuable knowledge throughout the process. On the other hand, Biotech startups need to work within constraints. One common option is to work with a consultant until the company is ready to hire. Before you decide when to hire a CMC consultant, take the following factors into careful consideration.
As You Bring Your Project Forward, You Must Develop the Chemistry, Manufacturing and Controls (CMC) Section of Your Process (and IND)
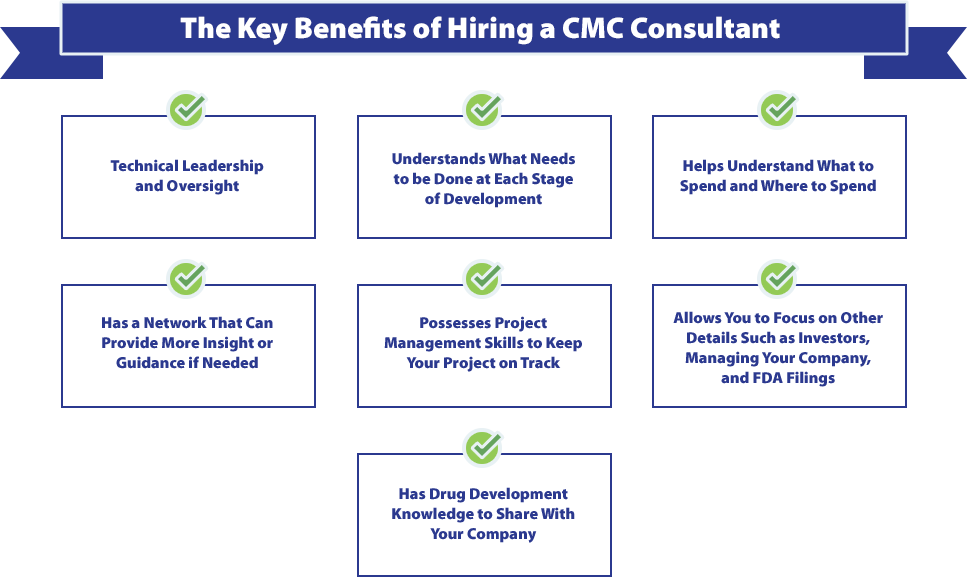
If your project is intricate and considered high stakes, you might want to consider working with a CMC consultant to further guide you. Having a veteran on your side can help anticipate issues and make more informed decisions. Working with someone who is highly qualified and possesses ample knowledge about the drug development process is critical. The right CMC consultant can guide you through each step of your project while also taking on necessary responsibilities.
These responsibilities should include:
- Writing requests for proposals (RFP).
- Making contact with qualified CDMOs (Contractors).
- Evaluating proposals from the CDMO or CRO.
- Overseeing technical components of the project.
- Managing contracts.
- Ensuring technical packages meet FDA standards.
- Providing technical oversight and project management.
Many biotech companies are started by experts in medicine and biotech research, yet Drug Development, Process Development, Scale-up and CGMP manufacturing require a very different skill set. Keep this in mind as every company will need to establish their own strategy at each stage of development for how they intend to meet regulatory requirements while also staying within a set budget and timeline. The chosen strategy should fit the program’s needs by keeping your end goal in mind (i.e., positioning the IP for sale, fast-tracking a potential blockbuster, or taking a step-by-step approach). This is critical as, for many startups, the company’s fate may rely on demonstrating pre-clinical or clinical results within a set amount of time.
There are many factors to consider when it comes to deciding whether to hire a CMC consultant for your project. If you are on the fence, start by asking yourself:
Do I have all of the required skills to oversee a CMC development project?
Am I covered form a regulatory, supply and technical perspective?
Before Hiring a CMC Consultant, Consider the Scale, Stage of Development, and Complexity of Your Project
A company may be able to handle the design and workload of a less-complex project (especially during the very early stages of development), but as a project progresses or when comes to a larger, more complex projects, a company may want to consider a bringing on a CMC consultant sooner than later.
For example, let’s say that a company is making hundreds of grams of a NCE (new chemical entity) to develop the synthesis. The company has experience in medicinal chemistry and in medical practice. The goal is to demonstrate proof of concept that the process works and deliver pre-clinical drug substance quantities for pre-clinical toxicology studies. In this case, the company may want to consider working with a small synthesis company or try to manage and plan out work on their own, in-house. In either case, and considering on the company’s confidence working with the small synthesis company, a CMC consultant may ensure that the team is working towards an efficient, scalable, pharmaceutically-appropriate, and transferrable process while helping the company avoid known hazards and pitfalls. The more representative the drug substance impurity profile is of the future CGMP campaigns, the less reworking will be needed to evaluate changes in the toxicology profile which will save the company time and money. This concept is further discussed in a previous blog post “Are Offshore CROs the Best Choice for Initial Drug API Quantities? – Regis Technologies”.
When this same company is preparing to make 1-10Kg of API for phase I/II, the goals are to demonstrate proof-of-concept at scale, and deliver API suitable for use in human trials under an IND. The need to have experienced CMC oversight grows substantially. There are many factors to consider that are not obvious to even the smartest scientific mind. Unless someone has experience in drug development, he or she may not understand and anticipate needs for process development, optimization, formulation, supply chain, regulatory and more. Companies may require quantities that far exceed what is being used in the clinic. Drug substances and drug products have long lead times to produce, meaning that being caught shorthanded on supply can have huge impact on timelines.
Drug Development Projects are Large, Complex Projects
Drug development projects are highly complex come with significant risk which proposes a serious challenge to every company. Due to strict regulations, drugs must be proven to be safe and effective before they may be marketed. The process of collecting that data has multiple gates to pass through, each with its own set of requirements. Such projects are daunting for a small company to take on alone.
For example, suppose a company is working on a project that is moving into phase II of drug development. The company will likely need to examine the process and incorporate learnings from the previous campaign and perform some targeted process development. Certain analytical methods may require additional rigor to be qualified for use. Demand projection increases may require engineering changes to scale-up production. Regulatory starting materials may need to be improved, reordered, or resourced. Different drug product formulations may need to be examined to improve performance or stability. Impurity profiles must be examined, and decisions made on what level of impurity characterization data is required.
All the CMC data needs to be summarized and formatted into the electronic common technical document format (eCTD) then submitted to the FDA in an IND for permission to move forward. One false step can have costly consequences. An experienced CMC consultant understands these issues, helps manage these risks, and keeps the project on-track.
What to Look for When Hiring a CMC Consultant
If a company decides that they are ready to hire a CMC consultant for a project, they’ll want to make sure that they hire the right person for the job. There are six main factors that should be taken into consideration when hiring a CMC:
Pedigree: Check if the CMC consultant has a track record taking molecules from IND to NDA and approval in the emerging pharma space. Make sure the CMC consultant is fully committed to supporting your project and your company.
Comprehension: A CMC consultant should understand the technical areas of your project (chemistry, analytical, route of delivery, etc.), the regulatory requirements and your project’s goals.
Location: Although a CMC consultant doesn’t have to be located in the same city as your company, you may want to consider time zones for the consultant, project team, and supply chain for effective communication.
Stewardship: The CMC consultant works for your company, not for the CDMO or other contractors.
Alignment: The CMC consultant should be someone that can relate to your project and company and can come up with a strategy that fits your needs.
Confidence: Your CMC consultant should be confident to voice an opinion different than yours and to present that to your team and/or manager.
Conclusion
Hiring a CMC consultant early can be extremely beneficial because drug development is highly regulated and extremely complex. Ask yourself, “does your company have all of the required skills to oversee a CMC development project?” If the answer is “no”, you should fill the gap with a CMC expert to oversee your project and guide you throughout the process.
Please contact us if you would like to learn more about partnering with Regis Technologies Inc. on your next project. A member from our team would welcome the opportunity to speak with you and provide insight on how we can be of value during your project.