Innovation Unleashed: Case Studies in Process Development by Regis Technologies


Introduction
Welcome to the third part of our process development blog series. If you haven’t had the chance to read the first and second parts of this series, The Role of Process Chemistry and Process Development: A Systematic Approach, you can do so by visiting our blog which is a great resource for those interested in the latest innovations and solutions within our industry.
In this article, we summarize three distinct case studies to show the impact of process development and the results that were achieved by the organizations that employed this method. In each case study, we will explain the unique challenges each organization faced, along with the strategies we leveraged to overcome them. Through the use of these case summaries, we intend to highlight why a systematic approach to process chemistry and development matters, and also how it can result in significant advancements in our field.
Case Study #1: Salt of the Earth
The primary objective of the inaugural case study, “Salt of the Earth,” was to engage Solid State Chemistry to solve a common problem associated with the scale-up of chemical processes. At the beginning of the case study, the first tox batch was produced successfully, with process development initiated before the first GMP campaign for P1 clinical studies. During development, numerous experimental runs were performed before lab confirmation, including checking that consistent yields and intermediate purity led to reproducibility.
During the lab confirmation batch, the yield increased significantly, and the purity remained consistent. The data showed no significant variance in purity from one lot to another, but the weight assay was observed to be lower when compared to earlier lots. NMR analysis by 1H and 13C showed no unexpected peaks. Based on the analysis, the reason for the drop in weight assay appear to be organic in nature.
The product of this step is isolated as a crystalline salt and has been shown to display only one characteristic powder pattern as determined by X-ray powder diffraction (XPRD). Upon analyzing lower weight assay while the same signature pattern was identified, several additional peaks were noted in the diffractogram. These extraneous peaks were consistent with the characteristic diffraction pattern of sodium chloride (NaCl), based on an in-house reference sample preparation.
During the workup/isolation process, NaCl was used in a saturated brine solution during the workup to aid in removing a polar solvent during extractive workups. When that saturated brine was switched to a more dilute NaCl wash at a higher temperature, the extraneous peaks attributed to NaCl in the powder diffraction pattern disappeared and the overall weight assay increased in a consistent, reproducible fashion. With a product of consistent quality in hand, the chemistry of the next step was successful and reproducible.
Case Study #2: The Solution is Crystal Clear
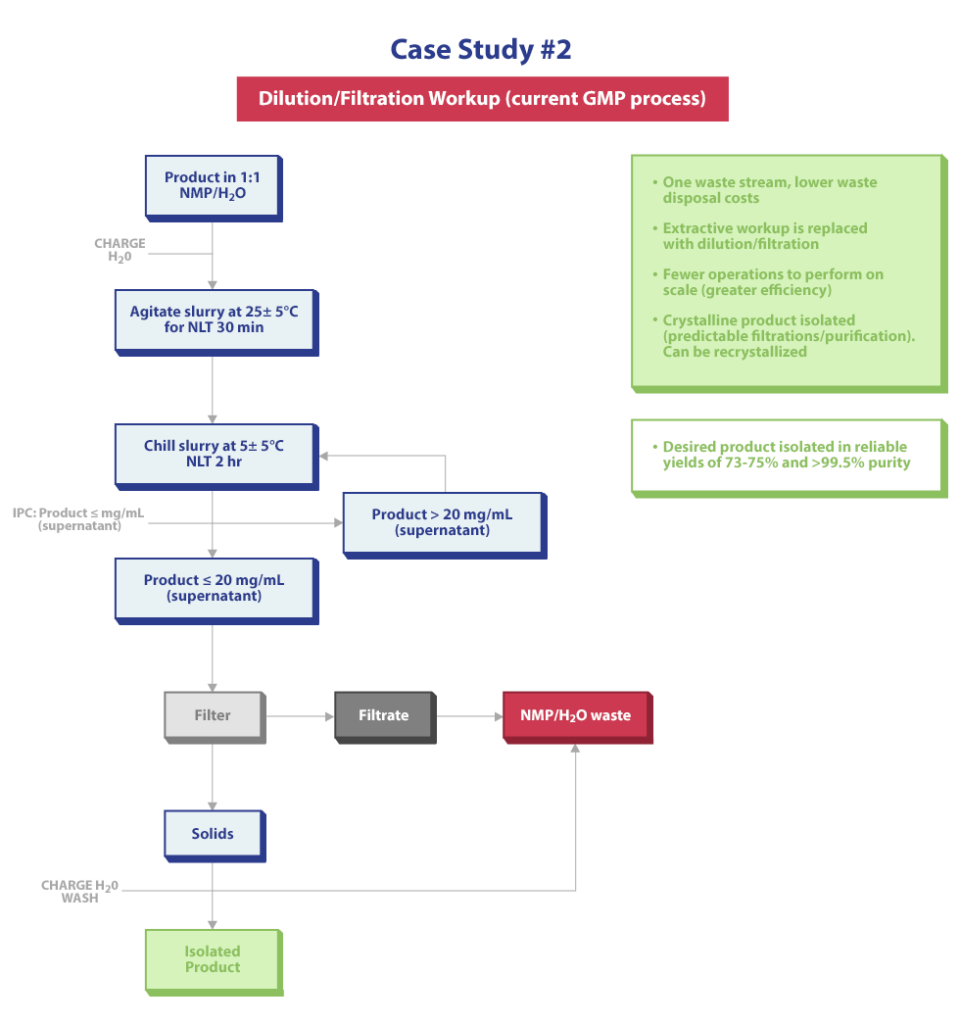
In our second case study, “The Solution is Crystal Clear,” the procedure provided to Regis Technologies (via Technical Transfer) was investigated by our team in the lab. In the original procedure, a reaction was completed in a 4:1 mixture of NMP/water. After reaction completion and filtration to remove solid by-products, multiple extractions of the filtrate into isopropyl acetate (IPAc) were required to maximize yield generating several waste streams. During familiarization and early development, it was found that after several extractions, the isolated product was amorphous.
The Process Chemistry team identified numerous problems during the development phase. First, the process did not perform reproducibility in terms of product quality/yield when scaled up. Second, the generation of multiple waste streams would not be desirable on plant manufacturing scale. Third, the amount of extractions/unit operations required to perform this process on scale was deemed prohibited. Fourth, a concentration of the organic (IPAc) layer to near dryness followed by an addition of water as an anti-solvent would afford a typically amorphous product that would vary in overall quality (purity).
During routine process development work, the team found that by changing the reaction mixture to a 1:1 mixture in NMP/water (reduced from 4:1 NMP/water), the desired product would start to precipitate out of the reaction mixture in a crystalline form and previously isolated amorphous material could even be recrystallized from NMP/water mixtures to the same crystalline form.
A modified workup procedure after reaction completion in 1:1 NMP/water greatly reduced unit operations and all process waste converged to one waste stream. Batch-to-batch variability in yield and quality were also greatly reduced by isolating a consistent crystalling product.
Case Study #3: Million Dollar Wash
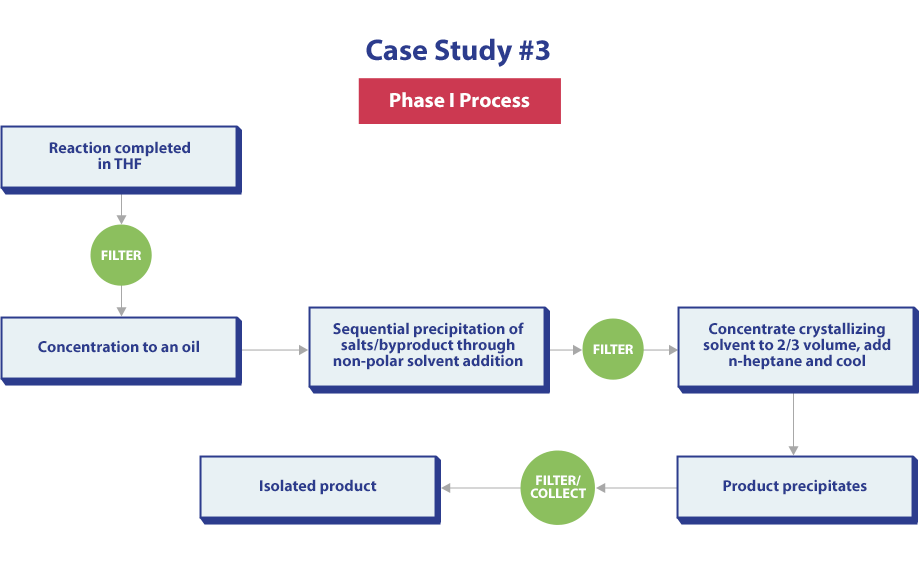
The final case study conducted was “Million Dollar Wash.” The Enabling Route described herein was initially developed to access a final API for early phase clinical studies, focusing strongly on time and expediency.
Phase 1 Process Steps:
- Step 1: Reaction Completed in THF
- Step 2: Concentration to an Oil
- Step 3: Sequential Precipitation of Salts Through MTBE Solvent Addition
- Step 4: Concentrate Crystalizing Solvent to ⅔ Volume
- Step 5: Product Precipitates
- Step 6: Isolated Product
While providing product of acceptable quality in a timely fashion, several problems were noted in this route. First, concentrations to oils should be avoided whenever possible, as these operations are difficult to scale up and concerns about product stability/consistency during performance of these operations can’t be ignored. Second, of the three filtration unit operations described in this process, two of them are required to remove salt by-products. Third, major variances in yield (range:25-44%) were observed over several manufacturing batches, attributed to spontaneous co-precipitation of desired product with salt by-product.
Understanding the solubility characteristics of the known salt by-products allowed the development team to make a simple switch from THF (water-miscible) as a reaction solvent to 2-MeTHF (largely water-immiscible) before Phase 2. This change now allowed water-soluble salts that had precipitated during the course of the reaction to be removed with water washes of the organic layer. The hydrophobic nature of the product also ensured complete retention in the organic layer. The workup process adapted for Phase 2 is summarized below:
Phase 2 Process Steps:
- Step 1: Reaction Completed in 2-MeTHF
- Step 2: Organic Stream Washed With Water
- Step 3: Washed Organic Stream Concentrated to ⅓ of the Starting Volume
- Step 4: Charge n-heptane
- Step 5: Cool Slurry to 5-10°C, Then Hold For 1-2 Hours
- Step 6: Collect Product
The process changes implemented for Phase 2 saw dramatic improvement in isolated yield (with no decrease in weight % assay compared to earlier lots). The yield range improved to 73-75% over 4 executed GMP-manufactured batches, doubling the API throughput for the same input of raw materials.
Conclusion
Industrial chemical process development requires a commitment to developing reproducible, safe, and robust chemical processes, while leveraging a dynamic coordination of an interdisciplinary team of subject matter experts. You must keep the end game (GMP Manufacturing and scalability) in mind when it comes to process development and remember that early engagement/collaboration is linked to higher ROI and a greater understanding of chemical processes.
If you would like to learn more about how our team of professional process chemists can assist your company in bringing a new drug to market, please don’t hesitate to contact us. A member of our team would be glad to learn more information about your company and discuss how we could help you throughout the drug development process.